Australian Office Of Gene Technology Document: "Dealings Involving Intentional Release" Explains Covid-19 "Vaccines" RELEASE GMOS INTO THE ENVIRONMENT!
Document Subtitle: Details on the applications and licences for Dealings involving an intentional release (DIR) of a GMO into the environment.
This is pretty scary and unacceptable.
Get the official government document here
Lets get into it - this is important.
IOJ clicked the filter of only medical GMO’s and, TA-DA, we find AstraZenica and other alleged vaccines which “INTENTIONALLY RELEASE GMO” into our environment.
Now a while back we sued the State of Costa Rica for protection of the environment due to the alleged vaccines shedding or “releasing into the environment”. We were shut down at the gate without even getting to be heard.
Now, with this concrete government evidence, we will have the opportunity to go back and challenge the covid vaccines on the basis of GMO release into the environment and prove to our judge its a “gene technology” in the OFFICE OF GENE TECHNOLOGIES.
Please remember our motto is BE THE PERSISTENCE!
This environmental shedding issue of release GMO’s into the environment is a grand experiment that must be stopped!…
So yes, IOJ will harp on this angle, like a dog with a bone, in front of a judge.
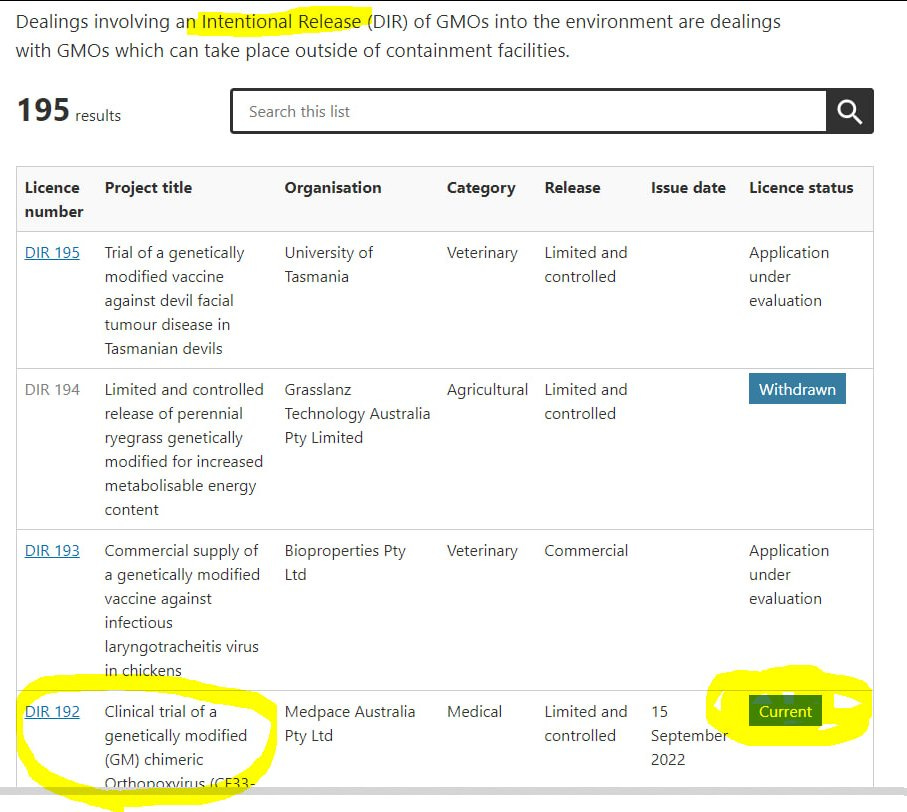
Below is the filtered list so you can see Australias approved intentional release of medical GMO’s into the environment using the biological agent covid-19 vaccine:
Here are a few choice results involving the covid-19 alleged vaccines from the Australian Government Office Of Gene Technology:
Some are ongoing GMO intentional releases since 2016!
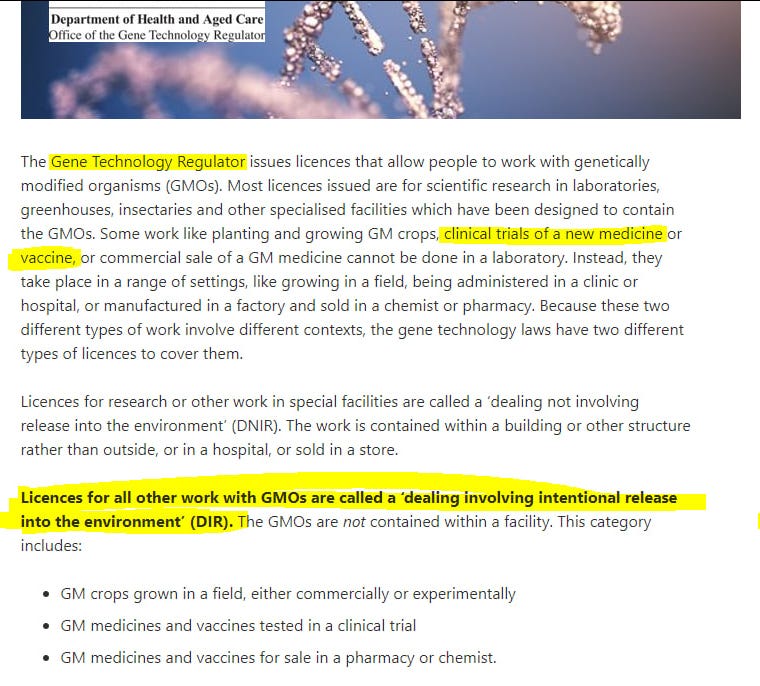
Dealings involving an Intentional Release (DIR) of GMOs into the environment are dealings with GMOs which can take place outside of containment facilities.
24 results total (below are just a few)
Commercial supply of attenuated GM influenza vaccines
AstraZeneca Pty Ltd
Medical Commercial14 January 2016 Current
Commercial supply of a genetically modified COVID-19 vaccine
AstraZeneca Pty Ltd
Medical Commercial8 February 2021 Current
Clinical trial of live attenuated genetically modified influenza vaccines
Novotech (Australia) Pty Ltd
Medical Limited and controlled1 August 2016 Current
Clinical trial of genetically modified influenza vaccine (H3N2 M2SR)
Novotech (Australia) Pty Ltd
Medical Limited and controlled10 June 2020 Current
A genetically modified respiratory syncytial virus (RSV) vaccine for use in clinical trials
Novotech (Australia) Pty Ltd
Medical Limited and controlled16 July 2018 Current
Commercial supply of a genetically modified COVID-19 vaccine
Janssen-Cilag Pty Ltd
Medical Commercial19 April 2021 Current
Clinical trial with a genetically modified human adenovirus COVID-19 vaccine
Avance Clinical Pty Ltd
Medical Limited and controlled25 June 2021 Current
Because Costa Rica uses AstraZenica, IOJ refers to the detailed information of AstraZenica GMO Intentional Release:
DIR 180
Commercial supply of a genetically modified COVID-19 vaccine
Licence details
Licence number: DIR 180
Licence status: Current
Category: Medical
Release: Commercial
Organisation: AstraZeneca Pty Ltd
Parent organism: Adenovirus
Modified trait: Vaccine - altered antigen expression; Vaccine – replication incompetent
Licence issue date: 8 February 2021
Description: This licence allows AstraZeneca Pty Ltd to import and distribute their COVID-19 vaccine as part of its commercial supply as a vaccine in Australia.
The Therapeutic Goods Administration (TGA) has responsibility for assessing the quality, safety and efficacy of any vaccine intended for use in people in Australia.
Once approved for use in Australia, products are registered by the TGA and can then be distributed. You can find out more about the TGA approval of the AstraZeneca vaccine on their website.
BOOM
The Regulator has not imposed any specific measures to manage risk because there is negligible risk to the health and safety of people or the environment.
Supply of the vaccine will follow the Australian code of good wholesaling practice for medicines in schedules 2, 3, 4 & 8 (2011), the WHO’s Good Distribution Practices for pharmaceutical products (WHO 2010), the National Vaccine Storage Guidelines: Strive for 5 (2019) and the Standard for the Uniform Scheduling of Medicines and Poisons (as current) which all provide appropriate controls and informed the risk assessment. Any unused vaccine or waste material will be disposed of in accordance with local requirements for clinical waste.
The risk analysis for this application was carried out in accordance with the Regulator’s Risk Analysis Framework.
Date application received: 7 December 2020
RARMP open for public consultation: 18 December 2020.
RARMP closed for public consultation: 18 January 2021
Documents
Licence decision - notification
Outlines the Regulator’s decision to issue a licence following the assessment of this application.
Download DIR 180 asWord - 51.3 KB
Download DIR 180 asPDF - 148.54 KB
Licence decision - Q&A
FAQs on the licence application and the Regulator’s decision to issue a licence for this application.
Download DIR 180 asWord - 27.32 KB
Download DIR 180 asPDF - 173.37 KB
Risk assessment and risk management plan - summary
A summary of the Risk Assessment and Risk Management Plan prepared as part of the assessment of this application. It provides a brief description of the licence application, the risk assessment and risk management plan.
Download DIR 180 asPDF - 243.69 KB
Download DIR 180 asWord - 59.06 KB
Risk assessment and risk management plan - full version
The final risk assessment and management plan prepared to support the Regulator's decision. It describes the GMO(s) and proposed work with the GMO(s) and provides an assessment of potential risks posed by the GMO(s). It also includes a summary of submissions received during the public consultation process.
Download DIR 180 asWord - 1.66 MB
Download DIR 180 asPDF - 1.74 MB
Licence conditions
Sets out the licence conditions imposed by the Regulator, including the licence holder’s general and specific obligations and reporting requirements.
Download DIR 180 asWord - 127.93 KB
Download DIR 180 asPDF - 301.49 KB
Last updated:
8 December 2021
Yikes!
Supply of the alleged vaccine in the gene technology office is supposed to follow the:
Australian code of good wholesaling practice for medicines in schedules 2, 3, 4 & 8 (2011),
the WHO’s Good Distribution Practices for pharmaceutical products (WHO 2010),
the National Vaccine Storage Guidelines: Strive for 5 (2019)
and the Standard for the Uniform Scheduling of Medicines and Poisons (as current) which all provide appropriate controls and informed the risk assessment.
There will need to be independent studies and a worldwide moratorium to protect the environment from intentional release of GMO. Rest assured that when our case goes to international court soon it will be a key topic.
If you think real court action must be taken to stop all countries from intentionally releasing GMO’s please support the legal fund so we can Sue The WHO and all their corrupt regulatory members such as TSA.
No one we know is willing to take this on in court in such a huge way and it must be done!






https://geneticliteracyproject.org/2020/07/17/european-union-suspends-some-gmo-regulations-to-fast-track-covid-19-vaccine/
https://rumble.com/v2mbo8c-david-martin-eu-parliament-international-covid-summit-may-3-2023-coronaviru.html