Arcturus Therapeutics Gains FDA Approval to Launch mRNA Clinical Trial for H5N1 Pandemic Flu Vaccine
This could be very much a GIANT business over the entire globe if we don't stop it! Could the W.H.O.'s amendments to the IHR and also Pandemic treaty be pre-planned for a global Pharma mRNA lab setup?
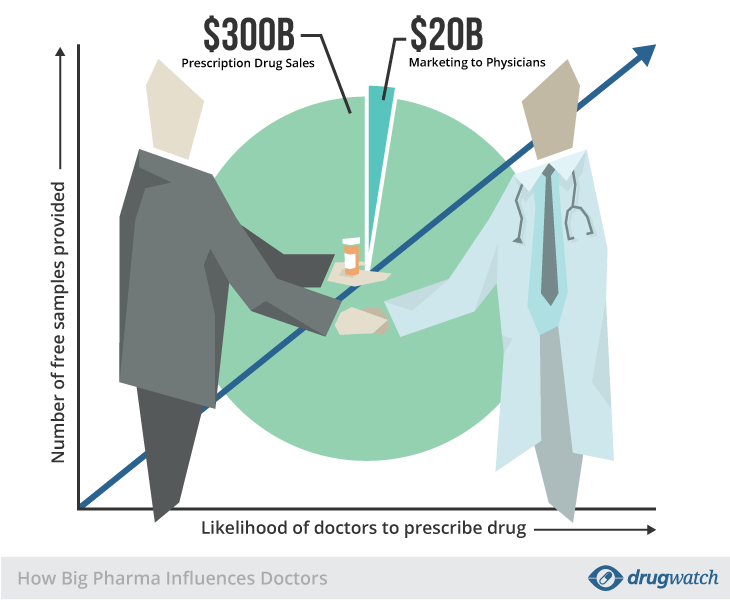
WOW! This is why IOJ has been on the WHO’s case as well as the FDA’s - Regulatory failure was a BIG part of the cause for the COVID-19 non vaccines being a global catastrophe during the “plandemic”. There is zero oversight over the Emergency Use Listing EUL, pre-qualification unit and the regulating of products on the global market like mRNA [non] vaccines.
This is one of the disputes IOJ has been harping on the Costa Rica government about during the 11-11 Nuremberg Hearing with Dr. Michael Yeadon, Dr. Janci Lindsay, Sasha Latypov, and others. The fact that Costa Rica’s regulatory agency called CONIS also failed to stop the experimentation on their own people including tricking the indigenous into taking it. IoJ has Supreme court testimony from the health minister of Costa Rica that the country relied all on WHO’s recommendations.
“Arcturus is actively engaged with the U.S. government to prepare for the next pandemic, and clearance to proceed into the clinic with our STARR® self-amplifying mRNA technology is a key step in this important process”
These types of Big Pharma Holding companies like Arcturus Therapeutics Holdings Inc., is the next step for Big Pharma on a global level, and if they get their way with the Pandemic Treaty in May 2025 during the 78th Word Health Assembly (WHA78), it will correlate with the WHO’s amendments to the International Health Regulations (IHR), allowing BIG Pharma business to prosper with their peddling of pandemic products on the global frontier.
Join our newly forming community where we are hosting webinars on how to stop the WHO Pandemic treaty and the amendments to the IHR - and also expect other wonderful surprises of information. If you join as a paid subscription, IOJ will be offering extra goodies and also Q&A’s with special guests ~
Source: SAN DIEGO--(BUSINESS WIRE)--
Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a commercial messenger RNA medicines company focused on the development of infectious disease vaccines and opportunities within liver and respiratory rare diseases, today announced that the U.S. Food and Drug Administration (FDA) has issued a “Study Can Proceed” notification for the Company’s Investigational New Drug (IND) application, ARCT-2304, a self-amplifying mRNA (sa-mRNA) vaccine candidate for active immunization to prevent pandemic influenza disease caused by H5N1 virus. The clinical study is funded by Biomedical Advanced Research and Development Authority (BARDA) and designed to enroll approximately 200 healthy adults in the United States.
“Arcturus is actively engaged with the U.S. government to prepare for the next pandemic, and clearance to proceed into the clinic with our STARR® self-amplifying mRNA technology is a key step in this important process”
“Arcturus is actively engaged with the U.S. government to prepare for the next pandemic, and clearance to proceed into the clinic with our STARR® self-amplifying mRNA technology is a key step in this important process,” said Joseph Payne, President & CEO of Arcturus Therapeutics. “The Phase 1 clinical trial is designed to evaluate the safety, reactogenicity, and immunogenicity of ARCT-2304 as a potential vaccine to protect against the highly pathogenic H5N1 avian influenza.”
About ARCT-2304
ARCT-2304 is a sa-mRNA vaccine candidate formulated within a lipid nanoparticle (LNP). The sa-mRNA vaccine candidate is designed to make many copies of mRNA within the host cell after intramuscular injection to achieve enhanced expression of haemagglutinin (HA) and neuraminidase (NA) antigens, thereby enabling lower doses than conventional mRNA vaccines. Utilizing a mRNA-based platform for pandemic influenza vaccine development offers further options for meeting domestic vaccine manufacturing surge capacity goals. The technology may make vaccines available much sooner than egg- and cell-based technologies. The lyophilized vaccine formulation is stable in refrigerators, thereby simplifying cold-chain storage and reducing distribution risks.
About Arcturus
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a commercial mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR® mRNA Technology (sa-mRNA) and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus developed KOSTAIVE®, the first self-amplifying messenger RNA (sa-mRNA) COVID vaccine in the world to be approved. Arcturus has an ongoing global collaboration for innovative mRNA vaccines with CSL Seqirus, and a joint venture in Japan, ARCALIS, focused on the manufacture of mRNA vaccines and therapeutics. Arcturus' pipeline includes RNA therapeutic candidates to potentially treat ornithine transcarbamylase (OTC) deficiency and cystic fibrosis (CF), along with its partnered mRNA vaccine programs for SARS-CoV-2 (COVID-19) and influenza. Arcturus' versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, circular RNA, antisense RNA, self-amplifying RNA, DNA, and gene editing therapeutics. Arcturus' technologies are covered by its extensive patent portfolio (over 400 patents and patent applications in the U.S., Europe, Japan, China, and other countries). For more information, visit www.ArcturusRx.com. In addition, please connect with us on Twitter and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding the initiation and enrollment of a Phase 1 clinical trial of ARCT-2304, the likelihood of success of the Company’s development and related efforts for an influenza vaccine candidate, the future activities under and fulfillment of the Company’s contract with BARDA, the ability of the Company’s influenza vaccine technologies to support U.S. government pandemic preparedness goals, and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the SEC, which are available on the SEC’s website at www.sec.gov. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Contacts
IR and Media Contacts
Arcturus Therapeutics
Neda Safarzadeh
VP, Head of IR/PR/Marketing
(858) 900-2682
IR@ArcturusRx.com
[END of Article]
Help IOJ in the fight for justice by making a substantial contribution. Help end unregulated products outside clinical trials such as the COVID-19 [non] vaccines. We must STOP unregulated experimental products from getting on the global marketplace!
IOJ will be continuing negotiations with the Costa Rica government and are on standby until further review of the evidence provided by the witnesses. We will keep you updated, join the community and we will be updating everyone as we go on the journey to Nuremberg.






I may be mistaken as I am going from memory but this company has violated the law with the covid injections (among other things).
David Martin knows their history very well and he is the go to person on this company, IMO.
The illness commonly known as influenza is merely one of a group collectively known as influenza-like illnesses (ILI). Despite propaganda to the contrary, they are neither infectious in nature nor are they contagious.
Given the absence of a submicroscopic, infectious particle called a “virus” as causative principle, obviously it’s not possible to “immunise” against it by mean of an injection.
There’s independent evidence that so-called “flu vaccines” do not reduce the incidence or severity of ILI episodes.
Now it’s necessary to look for alternative motivations for the development of such new products.
Using mRNA-based technology in their “flu vaccines” will axiomatically result in injuries, deaths & reductions in fertility in survivors. There’s your motive. Same as for “covid19” “vaccines”. Likely funded by the same sources, too.